
!
1!
!
!
Last!Modified:!September!2020!
Last!Reviewed:!February!2019!
Version:!1.0.1!
!
Protocol:!Optimization!of!lentiviral!transduction!using!
spinfection!!
!
Table&of&Contents&
&
1. Brief&Description………………………………………………………………………………………………………1&
2. Materials&and&Reagents…………………………………………………………………………………………….1&
3. Optimization&of&Spinfection&Conditions…………………………………………………………………….2&
a. STEP &1:&Cell&Density&and&Poly brene&Matrix…………………………………………………….2&
b. STEP &2:&Infec tion&Efficiency&and&Cell&Viability&Read&Out…………………………………5&
4. Polybrene&Sensitivity&Curve………………………………………………………………………………………7&
5. Perform&the&Spinfection&with&Another&Virus……………………………………………………………..9&
6. Example&Spinfection& C ondit ions……………………………………………………………………………..10&
7. Spinfection&Decision&Tree……………………………………………………………………………………….11&
!
&
1.&Brief&Description&
!
Lentiviral!transd u ction!is!an!effective!method!for!creating!a!stable!cell!line!with!a!DNA!cassette!of!
interest!integrated!into!its!genomic!DNA,!e.g.!an!sgRNA!or!gene!expression!cassette.!Transducing!
cells!at!a!concentrated!seeding!density!in!12-well!plates!while!centrifuging!(‘spinfection’),!is!a!
method!to!achieve!efficient!transduction!of!a!large!number!of!cells.! Cells!are!typically!infected!in!
the!presence!of!polybrene,!a!polycation!that!neutralizes!the!charge!repulsion!between!the!virus!
and!cell!target!surface!and!helps!viral!integration!into!the!cell.!!
!
The!ideal!spinfection!conditions!can! be!highly!variable!across!cell!types!and!sh ould!be! optimized,!
as!cell!lines!vary!in,!e.g ! infectability,!polybrene!sensitivity!and!response!to!dense!culture.!A!
balance!between!seeding!density,!polybrene!concentration!and!other!spinfection!variables!will!be!
determined!to!ensure!successful!transduction!and!minimal!cost!to!cell!viability.!This!protocol!uses!
the!pRosetta!GFP-vector!as!a!lentiviral!control!and!as!an!optional,!but !highly!recommended,!
simple!assessment!of!the!infectability!of!the!cell!line.!Transduction!efficiency!is!determined!as!the!
percentage!of!GFP-positive!cells,!via!f low!cyto metry.!!
!
2.&Materials&and&Reagents&
!
The!following!materials!are!required:!
!
• 30!million!cells!
• Growth!media,!PBS!and!trypsin!
!
2!
• Cell!counter!
• Trypan!Blue!(for!suspension!lines)!
• 12-well!plates!
• Tissue!culture!flasks!
• Polybrene!(Sigma-Aldrich,!Cat!#H9268,!make!a!stock!of!8!mg/mL!in!water,!store!at!-20C!
for!long-te rm!use,!keep!at!4C!for!2-3!weeks,!do!not!freeze/thaw!multiple!times)!
• Pipettes!
• 15!mL,!50!mL!tubes!
• 2!mL!of!pRosetta!(aka!pLKO_TRC060)!(PuroR,!BlastR,!GFP)!or!pRosettav2!(PuroR,!BlastR,!
HygroR,!GFP)!lentivirus!available!from!GPP!(gpp-reagents @broadinstitute.org)!!
!
The!following!materials!are!optional,!but!highly!recommended:!
!
• Flow!cyto meter!
• 96-well!V-bottomed!plates!
• Flow!buffer!(PBS,!2%!FBS,!5!uM!EDTA)!
!
!
3.&O ptimization&of&Spinfection&Conditions&
!
STEP &1:&Cell&Density&and&Polybrene&Matrix&
!
Perform!a!spinfection!using!a!range!of!cell!densities!and!polybrene!concentrations.!!
!
Day&1:& &
&
A:!Prepare!12-well!plates!and!master!mixes!
!
1. Label!two!12-well!plates!as!follows:!
!!!!!!!!
!!!!! !
!
2. Collect!the!cells:!
• For!ad h erent!cells:!
i. Aspirate! media!and!wash!flask!with!warm!PBS.!!
ii. Add!trypsin!and! allow!cells!to!detach !from!flask.!
0"PB"
NIC"
0"PB"
4"PB"
2"PB"
8"PB"
1.5E6"
1.##
2.##
3.##
4.##
5.## 6.## 7.## 8.##
9.## 10.## 11.## 12.##
0"PB"
NIC"
0"PB"
4"PB"
2"PB"
8"PB"
3E6"
1.##
2.##
3.##
4.##
5.## 6.## 7.## 8.##
9.## 10.## 11.## 12.##
!
3!
iii. Ad d!warm! media!(2-3x!the!amount!of!trypsin)!to!the!flask!t o!quench!the!
trypsin.!Transfer!the!cell!suspension!into!a!conical.!
iv. Wash!the!flask!with!media!and!add!the!wash!to!the!same!conical.!
v. Mix!the!cell!suspension!thoroughly!and!count.!!
• For!sus pension!cells:!
i. Collect!the!cell!suspension!from!the!flask!into!a!conical.!
ii. Sp in!down! the!cells!at!335-524!g!for!5!min!and!aspirate!
media!su pernatant.!!
iii. Re-suspend!the!cells!thoroughly!in!fresh,!warm!media!and!count.!
3. Dilute!the!cells! in!media!to!the!following!concentrations:!
a. 1.5E6!cells/mL!in!6!mL!to tal!(low-density)!
b. 3E6!cells/mL!in!6!mL!total!(high-density)!
4. Make!the!following!media,!8!mg/mL!polybrene!stock!and!pRosetta!virus!solutions:!
c. 3!mL!of!media!
d. 2.7!mL!media!+!0.3!mL!virus!
e. 2.7!mL!media!+!0.3!mL!virus!+!1.5!uL!polybrene!(final!=![2!ug/mL])!
f. 2.7!mL!media!+!0.3!mL!virus!+!3!uL!polybrene!(final!=![4!ug/mL])!
g. 2.7!mL!media!+!0.3!mL!virus!+!6!uL!polybrene!(final!=![8!ug/ mL])!
!
B:!Seed!cells!in!12-well!plates!
!
1. Add!1!mL!of!cell&suspension!and!1!mL!of!media/PB/virus&solution!to!the!corresponding!
labeled!wells,!as!follows:!!
!
Note:&the&wells&labeled&‘0&PB,&NIC’&are&the&no&polybrene,&no-infection&controls.&&
!
!!!!!!!Each!well!will!have!a!total!of!2!mL.!!
!
!!!!!!!!!!!!!! !!!!! !
!
C:!Centrifuge!th e!plates!
!
1. Spin!the!plates!at!931!g!for!2!hours!at!30°C.!!
!
!
!
!
0"PB"
NIC"
"a"+"c"
0"PB"
a"+"d"
2"PB"
a"+"e"
4"PB"
a"+"f"
8"PB"
a"+"g"
1.5E6"
1.##
2.##
3.##
4.##
5.## 6.## 7.## 8.##
9.## 10.## 11.## 12.##
0"PB"
NIC"
"b"+"c"
0"PB"
"b"+"d"
2"PB"
"b"+"e"
4"PB"
b"+"f"
8"PB"
"b"+"g"
3E6"
13.$$
14.$$
15.$$
16.$$
17.$$ 18.$$ 19.$$ 20.$$
21.$$ 22.$$ 23.$$ 24.$ $

!
4!
D:!Add!media!to!plates!and!incubate!
!
1. After!2!hours!of!spin n ing,!add!2!mL!of! media!(without!polybrene)!dropwise!on!top!of!each!
well.!!
2. Place!in!37°C!incubator!overnight.!!
&
Day&2:& !
&
E:!Inspect!cells!visually!
!
1. The!next!day!(16-24!hrs!later),!remove!the!plate!fro m! the!incubato r!and!ins pect!the!cells!
under!a!microscope.!!
2. Take!note!of!the!following!things:!
• If!the!cells!clumped!together!and!lifted!off!the!plate&
• The!confluency!of!the!wells!
• How!adhered!the!cells!are!to!the!plate!(suspension!cells!will!be!loosely!adherent)!
• The!amount!of!dead!cells!(typically!shriveled!and!floating)!
!
General' Assessment:''
'
• If!in!all!conditions:!
o The!cells!clumped!together!and!lifted!off!the!plate,!they!may!be!sensitive!to!a!high!
confluency!and/or!centrifugation.!Be!sure!to!gently!break!up!the!clumps!before!
counting!in!STEP&1,&Day&2,&Part&G.!!
o There!appear!to!be!a!lot!of!loosely!adherent!cells,!they!may!be!sensitive!to!density.!!
o There!appear!to!be!a!lot!of!dead!cells,!they!may!be!sensitive!to!centrifugation.!!
!
F:!Collect! the!cells!from!the!plates!
!
1. Collect!the!cells!from!the!plate:!
• For!ad h erent!cells:!
i. Label!10!conicals!with!the!condition!names!from!the!plate.!
ii. Collect!the!media!f rom!each!well!into!the!appropriate!conical.!
iii. Add!1!mL!of!PBS!to!each!well!and!collect!it!into!the!appropriate!conical.!!
iv. Add!250!uL!of!trypsin!to! each!well!and!let!the!cells!detach!from!the!plate.!!
v. Add!750!uL! of! media!to!each! well!to!quench!the!trypsin!and!transfer!the!cell!
suspension!to!the!appropriate!conical.!
vi. Add!1!mL!of!media! to!wash!each!well!and!transfer!the!wash!to!the!
appropriate!conical.!!
vii. Spin!the!conicals!at!335-524!g!for!5!min.!
viii. Aspirate!the!media!and!re-suspend!the!‘low-density’!cells!in!1!mL!of!fresh!
media!and!the!‘high-density’!cells!in!2!mL!of!medi a.!!
• For!sus pension!cells:!
i. Label!10!conicals!with!the!condition!names!from!the!plate.!
ii. Gently!pipette!the!media!in!each!well!up!and!down!to!dislodge!the!cells!and!
then!collect!the!cell!suspension!int o!the!appropriate!conical.!

!
5!
iii. Add!1!mL!of!media!to!wash!each!well!and!transf er!the!wash!to!the!
appropriate!conical.!!
iv. Spin!the!conicals!at!335-524!g!for!5!min.!
v. Aspirate! th e!media!and!re-suspend!the!lo w-density!cells!in!1!mL!of!fresh!
media!and!the!high-density!cells!in! 2!mL!of!media.!!
!
G:!Count!the!cells!and!assess!the!recovery!from!the!12-well!plates!
!
1. Cou nt!the!cells,!using! Trypan!blue!for!suspension!cells.!!
2. Record!yields!–!note&more&than&25%&loss&or&cell&deat h&in&any&condition.!!
!
General' Assessment:'
'
• Compare!the!recoveries!between!the!(-)polybrene,!(-)virus!wells!and!the!(-)polybrene,!
(+)virus!wells!(#1!vs.!#5!and!#13!vs.!#17):!if!the!numbers!are!lower! with!virus,!lentivirus!
may!be!toxic!to!the!cells.!'
• Compare!the!recoveries!between!the!(-)polybrene!wells!and!the!(+)polybrene!wells!(#5!vs.!
#s!6,!7,!8!and!#17!vs.!#s!18,!19,!20):!if!the!numbers !are!lower!with!polybrene,!polybrene!
may!be!toxic!to!the!cells.!'
• Compare!the!recoveries!between!the!‘low’!vs.!‘high-density’!wells!(ex.!#5!vs.!#17):!if!more!
cells!are!lost!from!the!‘high-density’!wells,!the!cells!may!be!sensitive!to!density.!'
'
H:!Seed!the!cells!into!flasks!!
!
1. Choose! appropriately!sized!flasks!based!on!cell!size!and!doubling!time!to!ensure!the!cells!
will!not!become!confluent!within!2-4!days.!
2. Label!the!10!flasks!with!each!12-well!condition!name.!!
3. Seed ! the!cells!from!each ! conical!into!the!corresponding!labeled! f lask!and! add!th e!
appropriate!volume!of!media.!!
4. Incubate!the!cells.!!
!
Day&4-6:&&
&
I:!Monitor!the!cells!post-spinfection!and!seeding!
!
1. Check!the!cells!under!the!microscope!over!the!next!2-4!days!and!note!any!significant!cell!
death!and/or!debris!in!any!of!the!conditions.!!
2. Before!the!cells!get!confluent,!stop!the!assay!and!continue!with!STEP&2.!!
!
&
STEP &2:!Infection&Efficiency&and&Cell&Viability&Read&Out!
!
Determine!the!infection!efficiency!and!cell!viability!via!several!methods.!!
!
A:!Visually!assess!the!confluency!and!health!of!the!cells!
!
1. Check!each!flask!under!the! microscope.!
!
6!
2. Take!note!of!the!following!things:!
• Cell!confluency!
• Morphological!changes!
• Amount!of!cell!death!and/or!debris!!
!
B:!Collect!and!count!the !cells!from!the!flasks!to!obtain!yields!
!
Note:&there&must&be&cells&left&over&from&each&flask&to&perform&flow&cytometry.&&
&
1. Collect!the!cells!from!each!flask,!ensuring!that!every!condition!is!kept!separate.!
2. Cou nt!the!cells!and!record!yields.!!
3. Save!the!cells!in!suspension!to!perform!flow!cytometry.!!!
!
C:!Optional,!but!highly!recommended:!assay!the!percentage!of!GFP-positive!cells!via!flow!
cytometry!
!
1. Take!200!uL!of!each!cell!suspension!and!seed!into!a!V-bottomed!96-well!plate!for!flow!
cytometry.!!
2. Add!100!uL!of!flow!buffer!(PBS,!2%!FBS,!5!uM!EDTA)!to!each!well!and!mix!by!pipetting!up!
and!down.!!
3. Assay!the!10! co nditions!via!f low!cytometry,!using!the!no-polybrene,!no-infection!control!
well!to!draw!the!appropriate!gates!for!GFP-negative!cells.!!
4. Record!the!%!GFP-positive!cells!for!each!condition,! indicating!the!percentage!of!infected!
cells.!!
!
General' A ssessment:'
'
• The!density!and!polybrene!combination!with!the!highest!yield!from!manual!counts!(and!
the!highest!%!viable!cells!from!Trypan!counts!for!suspension!cells)!and!the!highest!
percentage!of!GFP-positive!cells!will!likely!be!the!best! to!use!for!fu ture!spinfections.!!!
!
Troubleshooting'(also'see'the'Decision&Tree):'
'
• If!in!STEP&1,&Day&2,&Part&E!the!cells!clumped!and!lifted!off!the!plate!and/or!there!was!more!
than!25%!loss!AND!in!STEP&2,&Part&B&and&C!there!were!poor!yields!and/o r!low!GFP!
percentages,!the!cells!are!likely!sensitive!to!a!high!confluency!and/or!centrifugation.!
Repeat!the!protocol!trying!one!or!more!of!the!following!options:!!
o Continue! with!STEP&1&Day&2&Part&F ,!4-6!hours!post-spin!on!Day!1.!!
o A!no-spin!lentiviral!transdu ction!in!flasks!(see!Protocol:&No-spin&infection&for&
adhere nt&c ell&lines).!
o A!lower!seeding!density!of !8E5 !–!1E6!cells/12-well.!
!
• Notes!on!viral!toxicity:!
o If!there!is!a!significant!loss!of!cell!yield!when!com paring!the!(-)polybrene,!(-)virus!
flasks!to!the!(-)polybrene,!(+)virus!flasks,!lentivirus!is!likely!toxic:!
§ Use!a!low!range!of!virus!volumes!and!a!low!seeding!density!when!titrating!
with!another!virus.!
§ If!lentivirus!remains!toxic,!seek!an!alternative!method!of!delivery.!
!
7!
• Notes!on!comparing!po lybrene!concentratio ns:!
o The!highest!concentration!that!does!not!affect!cell!yield!should!be!used.!!
o If!there!is!a!significant!loss!in!cell!yield!at!all!concentrations,!polybrene!is!likely!
toxic.!Repeat!the!protocol!trying!one!or!more!of!the!following!options:!
§ Instead!of!adding!additional!media!on!top!of!the!cells!post-spin,!gently!
aspirate!the!media!from!the!wells.!Then,!dropwise!and!down!the!side!of!the!
well,!add!2!mL!of!fresh!media!on!top!of!the!cells!–!Note:!do¬&aspirate&
media&from&suspension&cells!&
§ Instead!of!incubating!th e!cells! overnight,!continue!with!STEP&1&Day&2&Part&
F,!4-6!hours!post-spin! on!Day!1.!!
§ A!lower!seed ing!density!of!8E5!–!1E6/12-well!and!no!polybrene!during!the!
spinfectio n.!
!
• Notes!on!comparing!low!vs.! high!density:!
o If!there!are!poor!infection!efficiencies!at!the!‘low!density’!(<25%),!the!cells!have!
very!low-infectability.!Repeat! u s ing!an!even! lo wer!seedin g!density!of!8E5!–!
1E6/12-well.!!
o If!there!are!poor!infection!efficiencies!at!the!‘high!density’!(<25%),!the!cells!have!
low-infectability.!Use!the!‘low!density’!when!infecting!with!another!virus.!!
o If!there!is!not!a!significant!loss!in!cell!yield/infection!efficiency!with!the!‘high!
density,’!it!can!be!used!with!high-titer!viruses!–!Note:&this&is&especially&useful&for&
some&genome-wide&libraries.!!
!
!
4.&Polybrene&Sensitivity&Curve&
&
If!the!ideal!spinfection!conditions!for!the!cells!are!already!known,!a!polybrene!sensitivity!assay!
may!be!performed!instead!of!the!above!cell!density!and!polybrene!matrix.!!
!
Day&1:& &
&
A:!Prepare!a!12-well!plate!and!cell!suspensions!
!
1. Label!one!12-well!plate!as!follows:!
!
!
!
2. Collect!the!cells! at!the!desired!concentration!in!10!mL!of!media!total.!
3. Label!4!conicals !with!the!po lybrene!concentrations:!0,!2 ,!4,!8.!
4. Add!2!mL!of!cell!suspension!to!each! of! the!conicals.!
0"PB 2 PB 4"PB 8 PB

!
8!
5. Add!the!following!volumes!of!8!mg/mL !polybrene!s tock!to!the!corresponding!conicals!and!
mix!thoroughly:!
a. 0!
b. 0.5!uL!!(final!=![2!ug/mL])!
c. 1!uL!!(final!=![4!ug/mL])!
d. 2!uL!!(final!=![8!ug/mL])!
!
B:!Seed!cells!in!12-well!plate!and!centrifuge!
!
1. Seed ! each!cell+polybrene!sus p ension!into!the!co rresponding! 12-well.!!
2. Spin!the!plates!at!931!g!for!2!hours!at!30°C.!!
3. After!the!spin,!add!2 ! mL!of!media! (without!polybrene)!dropwise!on!top!of ! each!well.!
4. Place!in!37°C!incubator!overnight.!!
!
Day&2:& &
&
C:!Inspect!and!collect!the!cells!
!
1. Visually!inspect!each!well!and!note!any!significant!amount!of!cell!debris.!!
2. Collect!the!cells!from!each!well!into!a!labeled!conical,!count!(using!Trypan!blue!with!
suspension!cells),!and!reco rd!yields.!!
!
D:!Seed!the!cells!
!
1. Choose! an!appropriately!sized!well-plate!or!flask!based!on!cell!size!and!doubling!time!to!
ensure!the!cells!will!not!become!confluent!within!3-4!days.!!
2. Label!4!wells/flasks!with!the!p o lybrene!concentrations.!
3. Seed ! the!cells!from!each ! conical!into!the!corresponding!wells/flasks!and!add!the!
appropriate!volume!of!media.!!
4. Incubate!the!cells.!!
&
Day&5-6:&!
!
E:!Collect!and!count!the!viable!cells!from!each!well/flask!(using!Trypan!blue!for!suspension!cells).!!
&
General' A ssessment:''
'
• The!highest!concentration!of!polybrene!that!results!in!less!than!10%!cell!toxicity!compared!
to!no!polybrene!should!be!used.!!
• If!po lybrene!is!toxic!at!all!concentrations,!repeat!the!protocol!trying!one!or!more!of!the!
following!options:!
o Instead!of!adding!additional!media!on!top!of!the!cells!post-spin,! gently!aspirate!the!
media!from!the!wells .!Then,!dropwise!and!down!the!side!o f!the!well,!add!2!mL!of!
fresh!media!–!Note:!do¬&aspirate&media&from&suspension&cells!!
o Instead!of!incubating!the!cells!overnight,!continue!with!Day&2&P a rt&C,!4-6!hours!
post-spin!on!Day!1.!!
!
!
9!
5.&Perform&the&Spinfection&with&Another&Virus!
!
The!chosen!spinfection!conditions!can!now!be!applied!to!future!lentiviral!infections!with!other!
viruses!besides!the!pRosetta!control.!For!a!list!of!available!control!viruses!please!visit!the!GPP!
Web!Portal!or!email!gpp-reag ents@broadinstitute.org!regarding!pooled!libraries.!!
!
• Most!of!these!vectors!have!an!antibiotic!selection!marker!to!confirm!successful!integration!
of!the!vector!into!the!cell’s!genome.!The!puromycin!or!blasticidin!dose!for!selection!of!the!
cells!must!be!optimized!prior!to!infection:!
!
!Selection!drug!dosing! (see!Protocol:&Puromycin,&blasticidin&an d&hygro my cin&titra ti on)!
&
• A!commonly!used!vector!is!pXPR_111!(pLEX_311Cas9v2)!for!introducing!Cas9!into!cells.!
This!vector!produces!very!low!titer!virus,!and!it!can!therefore!be!challenging!to!obtain!a!
high!infection!efficiency.!Project!Achilles!has!a!high!success!rate!with!the!following!
spinfectio n!conditions:!
!
o 1.5E6!cells!per!12-well!
o 750!uL!of!pLEX_311Cas9v2!virus!per!12-well!
o Polybrene!concentration!is!cell!line!dependent!!
o Infect!several!wells!at!the!same!time!to!obtain!more!screenable!cells!faster!(with!
one!non-in fection!control!well!to!assess!infection!efficiency!and!complete!
blasticidin!selection)!
o Add!blasticidin!48!hours!pos t-spinfection!!
!
• Before!beginning! a!screen,!a!6-well!seeding!density!test!and!viral!titration!with!the!library!
virus!should!be!performed!first:!!
!
!Cell!Density!and!Doubling!Rate!assay!(see!Protocol:&Pooled&Screen&Viral&Titration)!
&
!Viral!titration!with!library!virus!(see!Protocol:&Pooled&Screen&Viral&Titration)&
!
&
&
&
&
&
&
&
&
&
&
&
&
&
&
&
!
10!
6.&Example&Spinfection&Conditions&
!
Here!are!some!example!sp infections!conditions!for!reference:!!
!
HA1E!–!very!fast!doubling!time,!with!high-titer!virus!
• 1.5E6!cells!per!12-well!!
• 4!ug/mL!polybrene!
• Collected!cells!out!of!12-wells!4-6!hours!post-spinfection!to! avoid!clumping!and!lifting!off!
of!plate!!
!
TC32!–!sensitive!to!polybrene,!moderate!infectabi lity,!with !high-titer!virus!
• Optimized!2E6!cells!per!12-well!!
• 2!ug/mL!polybrene!
• Aspirated!and!replaced!media!after !2!hour!sp in!
!
BE(2)C!–!low!infectability,!not!polybrene!sensitive,!with!low-titer!virus!
• 1.5E6!cells!per!12-well!
• 8!ug/mL!polybrene!
!
A549!–!high-titer!virus!!
• 3E6!cells!per!12-well!
• 4!ug/mL!polybrene!
!
!
!
!
&
&
&
&
&
&
&
&
&
&
&
&
&
&
&
&
&
&
&
&
&
!
11!
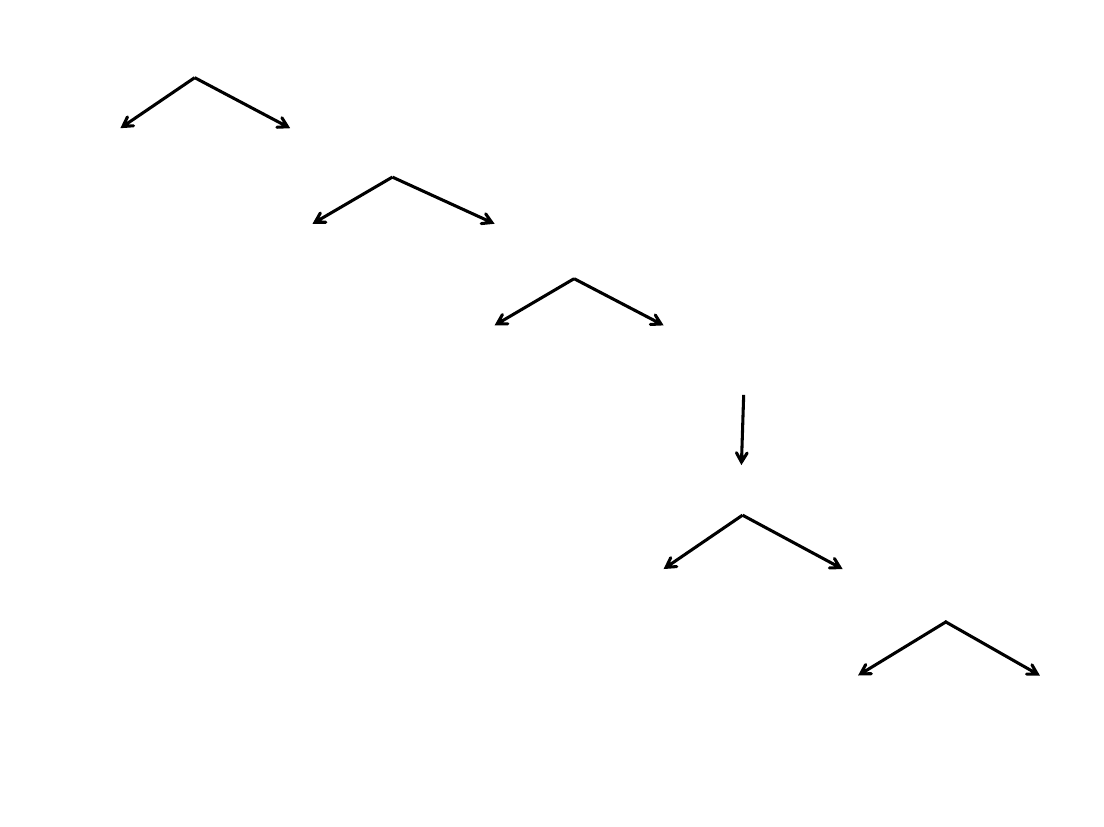
7.&Spinfection&Decision&Tree&
!
!
!
!
!
Did$the$cells$clump$or$die$
post-spinfection ?
Yes No
Try$these$tips:
Were$the$cells$infectable at$
the$low $d ensity?
No
• Collect'and'seed'
cells'4-6'hrs post-
spinfection
• No-spin'infection
• Seeding'density'of'
8E5'– 1E6
Were$the$cells$infectable at$
the$high$density?
YesNo
Use'the
low $d ensity
Was$lent i virus toxic to$
the$cells?
Yes No
• Use'a'low'range'of'
virus'volumes,'and'
the'low$dens i t y$for'
future'titratio ns
• Seek'alternative'
delivery'method
Was$polybrene toxic to$
the$cells?$
Yes No
Try$these$tips:
• Aspirate'and'replace'
media'post-spin
• Collect'and'seed'cells'4-
6'hrs post-spinfection
• Seeding'density'of'8E5'–
1E6,'no'polybrene
Pick'highest polybrene'
dose'that'does'not'
affect'cell'viability
Yes
Try$this:$
Seeding'density'
of'8E5'– 1E6
Piece'of'
cake!
